| Issue |
J Extra Corpor Technol
Volume 55, Number 4, December 2023
|
|
|---|---|---|
| Page(s) | 194 - 196 | |
| DOI | https://doi.org/10.1051/ject/2023036 | |
| Published online | 15 December 2023 | |
Technique or Application
Direct and continuous dosing of propofol can saturate Ex vivo ECMO circuit to improve propofol recovery
1
Utah Center for Nanomedicine, Department of Molecular Pharmaceutics, College of Pharmacy, University of Utah, Salt Lake City, Utah 84112, USA
2
Philipps Universität Marburg, Institut für Pharmazeutische Technologie und Biopharmazie, Robert-Koch-Straße 4, 35037 Marburg, Germany
3
Department of Biomedical Engineering, College of Engineering, University of Utah, 36 S. Wasatch, Salt Lake City, Utah 84112, USA
4
Division of Clinical Pharmacology, Department of Pediatrics, School of Medicine, University of Utah, 295 Chipeta Way, Salt Lake City, Utah 84108, USA
* Corresponding author: kevin.watt@hsc.utah.edu
Received:
30
March
2023
Accepted:
28
July
2023
Background: Extracorporeal membrane oxygenation (ECMO) is a cardiopulmonary bypass device that provides life-saving complete respiratory and cardiac support in patients with cardiorespiratory failure. The majority of drugs prescribed to patients on ECMO lack a dosing strategy optimized for ECMO patients. Several studies demonstrated that dosing is different in this population because the ECMO circuit components can adsorb drugs and affect drug exposure substantially. Saturation of ECMO circuit components by drug disposition has been posited but has not been proven. In this study, we have attempted to determine if propofol adsorption is saturable in ex vivo ECMO circuits. Methods: We injected ex vivo ECMO circuits with propofol, a drug that is highly adsorbed to the ECMO circuit components. Propofol was injected as a bolus dose (50 μg/mL) and a continuous infusion dose (6 mg/h) to investigate the saturation of the ECMO circuit. Results: After the bolus dose, only 27% of propofol was recovered after 30 minutes which is as expected. However, >80% propofol was recovered after the infusion dose which persisted even when the infusion dose was discontinued. Conclusion: Our results suggest that if ECMO circuits are dosed directly with propofol, drug adsorption can be eliminated as a cause for altered drug exposure.
Field of Research: Artificial Lung/ECMO
Key words: ECMO / Propofol / Saturation / Dosing
© The Author(s), published by EDP Sciences, 2023
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Overview
Extracorporeal membrane oxygenator (ECMO) is a cardiopulmonary bypass device that provides complete respiratory and cardiac support in patients with severe cardiopulmonary failure and is a lifesaving technology for critically ill patients. Critically ill patients on ECMO are prescribed multiple drugs but the dosing for most drugs is unknown. Dosing for this population is different due to 1) direct adsorption of drugs by the circuit, 2) clearance by the circuits (e.g., hemofiltration), and 3) critical illness physiology which affects drug absorption, distribution, metabolism, and elimination [1, 2]. Altered drug pharmacokinetics (PK) can result in suboptimal dosing and place patients at risk for subtherapeutic exposure and toxicity [3].
Adsorption of the drug to circuit components is primarily driven by hydrophobic and electrostatic interactions [4]. Studies have suggested that this process might be saturable but this has never been proven [5, 6]. Propofol is a commonly used sedative that is highly lipophilic (logP = 3.8) and protein-bound (98%) and is known to be adsorbed by the ECMO circuit. Prior studies have demonstrated that up to 70% of propofol was adsorbed within 30 minutes of administering a bolus dose to the ex vivo ECMO circuit [7–9]. In this study, we repeated the single dose experiment and additionally gave a 2-hour infusion of propofol to determine if propofol adsorption was saturable in a pediatric ex vivo ECMO circuit.
Description
ECMO circuits were assembled according to standard practice with a Quadrox iD oxygenator with bioline coating, Rotaflow R32 pump with bioline coating and custom perfusion tubing. Circuits were set up in a closed loop with an IV bag representing the “patient”. Circuits were primed with human-packed red blood cells (330 mL), fresh frozen plasma (150 mL), plasmalyte (100 mL), heparin (250 units), sodium bicarbonate (3.5 mEq), tromethamine (2 g), calcium gluconate (325 mg) and albumin (6.25 g) and adjusted to maintain physiological conditions. The flow was set to 1 L/min to mimic flows for a 10 kg child. For the control, 30 mL of primed blood was drawn from the ECMO circuit and transferred to a tube, and maintained at 37 °C in a water bath. Both the ECMO circuit and control were dosed with propofol at time 0 to achieve a target concentration of 50 μg/mL (for clinical relevance). Samples (1 mL) were collected at different time points (1, 5, 15, 30, 60, 120, 180, and 240 mins). After the 240-minute sample, the ECMO circuit was dosed with a continuous infusion of 6 mg/h representing the low end of dosing for a 10 kg child. The infusion was discontinued after 2 hours. Samples were collected at the following time points: 15, 30, 60, and 120 min after start of infusion and 1, 5, 15, 30, 60, 120, and 240 mins after discontinuing infusion. Collected blood samples were centrifuged immediately (@3000 rpm, 4 °C) and plasma samples were stored at −80 °C until ready for further analysis. The samples were then analyzed for propofol concentration using HPLC, and then converted to propofol remaining (propofol recovery) using the following equation: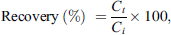 (1)where Ct is the concentration at time t and Ci is the concentration at time = 1 min for the bolus dose and Ci is the concentration at time = 15 mins for the infusion dose.
(1)where Ct is the concentration at time t and Ci is the concentration at time = 1 min for the bolus dose and Ci is the concentration at time = 15 mins for the infusion dose.
Results
The percentage recovery of propofol was then plotted against time (Figure 1). All circuits and controls were run in triplicate.
 |
Figure 1 Recovery of propofol at different time points. Bolus dose was given at t = 0 and infusion dose started at t = 4 h and stopped at t = 6 h with samples collected up to a total of 10 h. Propofol represents samples collected from the ECMO circuit and propofol control represents the samples collected from non-ECMO falcon tube controls. Data represent Mean (n = 3) ± SD. |
For the bolus dose, the control demonstrated a very high recovery of propofol (above 80% at all time points) whereas in the ECMO circuit, a rapid decrease in propofol recovery was observed after the bolus dose, with a recovery of only 27% at 30 mins (Figure 1 and Table 1). However, when administered as a continuous infusion, propofol recovery was >80% during the infusion and that high level of recovery persisted even after the infusion was discontinued.
Recovery of propofol at different time points.
Discussion
Our results following a bolus dose of propofol are consistent with previous investigations on recovery of propofol [7–9]. As noted in those studies, approximately 70% of propofol is adsorbed on the ECMO circuit components after 30 mins of a bolus dose. Our results were consistent with previous studies as we observed 72% propofol to be absorbed on the ECMO circuit components after 30 mins of a bolus dose. Our results following a continuous infusion suggest that propofol adsorption is saturable when administered through the circuit. The saturation of the circuit can occur due to the hydrophobic interactions between the hydrophobic drug propofol and the hydrophobic material of the ECMO circuit components. The tubing of the ECMO circuit is made of poly(vinyl chloride) (PVC) and the oxygenators are made of poly(methyl pentene) (PMP), which are both hydrophobic. By saturating the circuit, propofol will then be available for its primary purpose: sedation of the patient. However, it is difficult to directly translate ex vivo results to a clinical setting because the experiments only quantify the drug-circuit interactions and it is unknown if propofol administered directly to the patient would achieve the same saturation. To address this, we have developed an approach that uses physiologically based pharmacokinetic modeling (PBPK) to translate ex vivo results into dosing recommendations. One limitation of this study is that we set up the circuit as we would for a pediatric patient and hence results may not translate directly to an adult circuit. However, although we used a pre-configured pediatric tubing set and set the flow rate to 1 L/min, our ex vivo circuit had an adult oxygenator (common practice in pediatric ECMO centers). Because adsorption is dependent in part on flow rate in an ex vivo circuit, in order to translate this to an adult ECMO circuit, higher doses of propofol would be required. Another potential limitation is that propofol rapidly distributes out of the plasma when administered to a patient, and it is unknown if those low concentrations in the plasma would be sufficient to saturate the circuit. There are some concerns that administering a lipophilic drug directly to an ECMO circuit can increase clotting and impair oxygenator function [10]. Another unknown is the possibility of the circuit acting as a reservoir and leading to the leaching of the drug back into circulation and causing toxicity [5]. Consequently, additional ex vivo and in vivo work is required before dosing propofol directly to the circuit can be routinely recommended.
Conclusion
In summary, this is the first study to definitively show that adsorption by the ECMO circuit is saturable after an infusion dose is administered. Furthermore, these results demonstrate the potential of removing adsorption as a source of altered drug exposure by saturating the circuit. Additional work is needed to determine the optimal dose and if direct administration to the circuit impacts circuit function.
Acknowledgments
This research was conducted under a Pilot Award funded by the University of Utah Clinical and Translational Science Institute (CTSI).
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was funded by the University of Utah Clinical and Translational Science Institute Pilot Award program.
Data availability
The research data are available on request from the authors.
Author contributions
Nitish Khurana: Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Validation, Writing – original draft; Till Süenner: Investigation, Methodology; Carina Imburgia: Investigation, Methodology; Venkata Yellepeddi: Conceptualization, Funding Acquisition, Methodology, Writing – review and editing; Hamidreza Ghandehari: Conceptualization, Funding Acquisition, Methodology, Writing – review and editing, Supervision; Kevin M. Watt: Conceptualization, Funding Acquisition, Methodology, Writing – review and editing, Supervision.
References
- Sherwin J, Heath T, Watt K. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: a review of the current literature. Clin Ther. 2016;38:1976–1994. [CrossRef] [PubMed] [Google Scholar]
- Watt K, Li JS, Benjamin DK, Cohen-Wolkowiez M. Pediatric cardiovascular drug dosing in critically ill children and extracorporeal membrane oxygenation. J Cardiovasc Pharmacol. 2011;58:126–132. [CrossRef] [PubMed] [Google Scholar]
- Nolin TD, Aronoff GR, Fissell WH, et al. Pharmacokinetic assessment in patients receiving continuous RRT: perspectives from the Kidney Health Initiative. Clin J Am Soc Nephrol. 2015;10:159–164. [CrossRef] [PubMed] [Google Scholar]
- Preston TJ, Ratliff TM, Gomez D, Olshove VE, Nicol KK, Sargel CL, Chicoine LG. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol. 2010;42:199–202. [PubMed] [Google Scholar]
- Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–266. [CrossRef] [PubMed] [Google Scholar]
- Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–2116. [CrossRef] [PubMed] [Google Scholar]
- Lemaitre F, Hasni N, Leprince P, et al. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit Care. 2015;19:40. [CrossRef] [PubMed] [Google Scholar]
- Mulla H, Lawson G, von Anrep C, Burke MD, Upton DU, Firmin RK, Killer H. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21–26. [CrossRef] [PubMed] [Google Scholar]
- Hynynen M, Hammarén E, Rosenberg PH. Propofol sequestration within the extracorporeal circuit. Can J Anaesth. 1994;41:583–588. [CrossRef] [PubMed] [Google Scholar]
- Buck ML, Ksenich RA, Wooldridge P. Effect of infusing fat emulsion into extracorporeal membrane oxygenation circuits. Pharmacotherapy. 1997;17:1292–1295. [CrossRef] [PubMed] [Google Scholar]
Cite this article as: Khurana N, Sünner T, Hubbard O, Imburgia CE, Yellepeddi V, Ghandehari H & Watt KM. Direct and continuous dosing of propofol can saturate Ex vivo ECMO circuit to improve propofol recovery. J Extra Corpor Technol 2023, 55, 194–196
All Tables
All Figures
 |
Figure 1 Recovery of propofol at different time points. Bolus dose was given at t = 0 and infusion dose started at t = 4 h and stopped at t = 6 h with samples collected up to a total of 10 h. Propofol represents samples collected from the ECMO circuit and propofol control represents the samples collected from non-ECMO falcon tube controls. Data represent Mean (n = 3) ± SD. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


